A study led by the Indiana University School of Medicine in collaboration with the University of Chicago (United States) has presented Future possibilities for the treatment of type 1 diabetes and the possible reduction of insulin dependence.
The researchers’ findings, published in Cell Reports Medicine, suggest that lrepurposing the drug a-difluoromethylornithine (DFMO) can open doors to innovative therapies in the future.
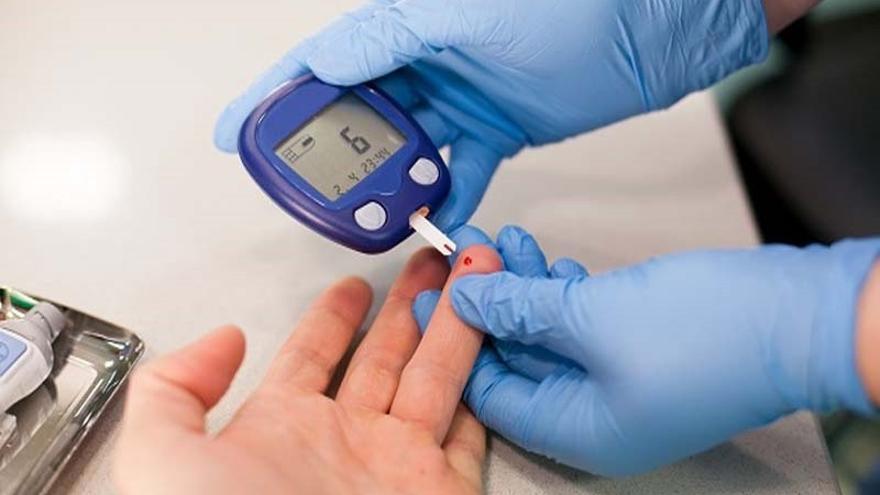
Type 1 diabetes is a chronic condition in which the body’s immune system mistakenly attacks and destroys the insulin-producing beta cells in the pancreas, causing high blood sugar levels that currently require insulin treatment. life to keep patients alive. Many people living with type 1 diabetes find that current treatments, including daily insulin injections and frequent blood sugar monitoring, They are uncomfortable and difficult to handle.
These latest translational results represent more than a decade of research. In 2010, the study’s corresponding co-author, Raghu Mirmira, was operating a research laboratory at the Indiana University School of Medicine in 2010 when his team initially discovered that Inhibition of the metabolic pathway affected by DFMO could protect to beta cells from environmental factors, suggesting possible preservation in type 1 diabetes. The team subsequently validated these findings in mice.
From 2015 to 2019, Edwin Letzter Professor of Pediatrics at Indiana University School of Medicine and pediatric endocrinologist and division chief at Riley Children’s Health Linda DiMeglio led a clinical trial that affirmed the safety of DFMO in newborns. diagnosed. with type 1 diabetes and suggested that it could also stabilize insulin levels by protecting beta cells. The trial was funded by the Juvenile Diabetes Research Foundation (JDRF) with a drug provided by Panbela Therapeutics.
A pill can replace injections
«After several years of comparative studies, starting with Dr. Mirmira and Tersey’s mouse models, it is exciting to finally be able to share the promising results of our pilot trial in humans,» said the study’s lead author, Linda DiMeglio. «Now that we have established the preliminary safety of DFMO for people with type 1 diabetes«We are excited to advance our collaborative research to explore more of its potential benefits in a larger clinical trial,» he added.
Related news
«The use of a new formulation of DFMO in pill form It allows patients to take it orally instead of having to undergo regular injections, and it has a very favorable side effect profile,» says Mirmir. «It’s exciting to say that we have a drug that works differently than any other treatment we have. we have for this disease,» he adds.
The researchers have already begun their next steps to investigate the potential of DFMO. The study’s first author and co-author, Emily K. Sims, recently released a Larger six-center clinical study to robustly define the impact of DFMO treatment on preserving beta cell function in type 1 diabetes.